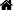ABSTRACT
Folliculogenesis is essential for production of female gametes in vertebrates. However, the molecular mech-anisms underlying follicle development, particularly apoptosis regulation in ovary, remain elusive. Here, we generated sox3 knockout zebrafish lines using CRISPR/ Cas9. sox3 knockout led to follicle development retar-dation and a reduced fecundity in females. Comparative analysis of transcriptome between sox3−/− and wild-type ovaries revealed that Sox3 was involved in pathways of ovarian steroidogenesis and apoptosis.
Knockout of sox3 promoted follicle apoptosis and obvious apoptosis signals were detected in somatic cells of stages III and IV follicles of sox3−/− ovaries. Moreover, Sox3 can bind to and activate the promoter of cyp19a1a. Up-regulation of Cyp19a1a expression promoted 17β-estradiol syn-thesis, which inhibited apoptosis in follicle develop-ment. Thus, Sox3 functions as a regulator of Cyp19a1a expression, via 17β-E2 linking apoptosis suppression, which is implicated in improving female fecundity.
INTRODUCTION
Qiang Hong and Cong Li are co-first authors.
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s13238-018-0603-y) contains sup-plementary material, which is available to authorized users.
cells. The communication between oocytes and granulosa cells is essential for oocyte development (Eppig, 2001). Granulosa cells can provide some substances, including cholesterol, specific amino acids and nutrients for oocyte development (Su et al., 2009), while oocytes secrete some paracrine factors, such as BMP15 (bone morphogenetic protein 15) and GDF9 (growth differentiation factor 9) to regulate granulosa cells development (Su et al., 2004; Su et al., 2009). Hence, the bidirectional communication between oocytes and somatic cells is important for oogen-esis.
Studies have shown that autophagy of ovarian somatic cells is involved in regulation of follicular development by maintaining cell homeostasis (Yuan et al., 2015), while apoptosis of granulosa cells is pivotal for follicular develop-ment through atresia of many early follicles to ensure growth and maturation of some dominant follicles (Matsuda et al., 2012). However, the molecular mechanisms connecting apoptosis in ovary to follicle development remain largely unknown. Identification of key regulators of apoptosis and relevant pathways is important for understanding ovarian functions.
In follicle development, some factors and relevant path-ways involved in both pro- and anti-apoptotic have been identified. FSH inhibited FoxO1-dependent apoptosis by coordinating the PKA-PI3K-AKT-FoxO1 axis and FoxO1-FoxO1 positive feedback in mouse granulosa cells (Shen et al., 2014). FGF-2 can inhibit apoptosis and promote follicle growth in culture in vitro in sheep (Santos et al., 2014).
Up-regulated apoptosis was associated with a decrease in number of vitellogenic follicles when the physiochemical waters parameters were unfavorable in fish (Thome et al., 2012). BCL2 family members, which included pro-apoptotic (e.g., Bax and Bok) and anti-apoptotic (e.g., Bcl2 and Mcl1) genes, were another kind of apoptosis regulators during follicle development (Perez et al., 1999; Hutt, 2015). Bcl2