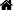“上海酶联文献” Wentao Fan a, Yanan Lv a, Shuai Ren a, Manyu Shao a, Tongtong Shen a, Kehe Huang a, Jiyong Zhou a, b, Liping Yan a, b, **, Suquan Song a,
aCollege of Veterinary Medicine, Nanjing Agricultural University, Nanjing 210095, Jiangsu Province, China
bJiangsu Engineering Laboratory of Animal Immunology, Institute of Immunology and College of Veterinary Medicine, Nanjing Agricultural University, Nanjing 210095, Jiangsu Province, China
highlights
ZEA increased NLRP3 inflammasome expression and cytokines release in cells. Elevated cytokines induced severe intestinal inflammation in ZEA-treated mice. ZEA induced colitis by activating ROS mediated NLRP3 inflammasome.
Article history:
Received 4 August 2017
Received in revised form
21 September 2017
Accepted 29 September 2017
Available online 30 September 2017
Handling Editor: A. Gies
Keywords:
Zearalenone
NLRP3 inflammasome Pro-inflammatory cytokines Intestinal inflammation Reactive oxygen species
a b s t r a c t
To ascertain whether zearalenone (ZEA) could induce intestinal inflammation and investigate its possible mechanism, we investigated inflammatory cytokine release and the activation of the NLRP3 inflamma-some after ZEA treatment both in vitro or in vivo. First, intestinal porcine enterocyte cell line (IPEC-J2) cells and mouse peritoneal macrophages were treated with ZEA to detect NLRP3 inflammasome acti-vation, and the role of reactive oxygen species (ROS) in ZEA-induced inflammation was investigated. Then, Balb/c mice were fed a gavage of ZEA, and the disease activity indices (DAIs) and histological analysis were used to assess intestinal inflammation. Our study showed that the mRNA
expression of NLRP3 inflammasome, pro-interleukin-1b (pro-IL-1b), and pro-interleukin-18 (pro-IL-18) was up-regulated 0.5- to 1-fold and that the release of IL-1b and IL-18 increased from 48 pg mL 1 to 55 pg mL 1 and 110 pg mL 1 to 145 pg mL 1, respectively. However, ROS inhibitor N-acetyl-L-cysteine (NAC) reduced IL-1b and IL-18 release to 45 pg mL 1 and 108 pg mL 1. Moreover, the same phenomenon was observed in intestinal tissues of ZEA-treated mice. In addition, clinical parameters of treated mice showed stools became loose and contained mucous. In addition, the presence of gross blood stool was found in the last 2 d.
Histological analysis showed obvious inflammatory cell infiltration and tissue damage in the colon. These findings uncovered a possible mechanism of intestinal mucosal innate im-munity in response to mycotoxin ZEA that ZEA could activate the ROS-mediated NLRP3 inflammasome and, in turn, contribute to the caspase-1-dependent activation of the inflammatory cytokines IL-1b and IL-18.
References
Ascenzi, P., Bocedi, A., Marino, M., 2006. Structureefunction relationship of estro-gen receptor a and b: impact on human health. Mol. Aspect. Med. 27, 299e402.
Bauer, C., Duewell, P., Mayer, C., Lehr, H.A., Fitzgerald, K.A., Dauer, M., Tschopp, J., Endres, S., Latz, E., Schnurr, M., 2010. Colitis induced in mice with dextran sulfate sodium (DSS) is mediated by the NLRP3 inflammasome. Gut 59, 1192.
Hankenson, Claire F., Braden-Weiss, Gillian C., Blendy, 2011. Behavioral and activity assessment of laboratory mice (Mus musculus) after tail biopsy under iso-flurane anesthesia. J. Am. Assoc. Lab. Anim. Sci. 50, 686e694 (689).
Camuesco, D., Galvez, J., Nieto, A., Comalada, M., Rodríguezcabezas, M.E., Concha, A., Xaus, J., Zarzuelo, A., 2005. Dietary olive oil supplemented with fish oil, rich in EPA and DHA (n-3) polyunsaturated fatty acids, attenuates colonic inflamma-tion in rats with DSS-induced colitis. J. Nutr. 135, 687.
Dinarello, C.A., 1996. Biologic basis for interleukin-1 in disease. Blood 87, 2095. Duarte, E.R., Oliveira, L.N., Oliveira, N.J.F.D., Abrao,~ F.O., Souza, R.M.D., Melo, M.M.,
2013. Concomitant zearalenone ingestion and porcine Circovirus-2 infection.
Acta Sci. Veterinari 41, 1e6.
Fan, W., Shen, T., Ding, Q., Lv, Y., Li, L., Huang, K., Yan, L., Song, S., 2017. Zearalenone induces ROS-mediated mitochondrial damage in porcine IPEC-J2 cells. J. Biochem. Mol. Toxicol. e21944.
Ferrer, E., Juangarcía, A., Font, G., Ruiz, M.J., 2009. Reactive oxygen species induced by beauvericin, patulin and zearalenone in CHO-K1 cells. Toxicol. Vitro Int. J. Publ. Assoc. Bibra 23, 1504e1509.
Finkgremmels, J., Malekinejad, H., 2007. Clinical effects and biochemical mecha-nisms associated with exposure to the mycoestrogen zearalenone. Anim. Feed Sci. Technol. 137, 326e341.
Harris, H.A., Albert, L.M., Leathurby, Y., Malamas, M.S., Mewshaw, R.E., Miller, C.P., Kharode, Y.P., Marzolf, J., Komm, B.S., Winneker, R.C., 2003. Evaluation of an estrogen receptor-beta agonist in animal models of human disease. Endocri-nology 144, 4241e4249.
Horwood, N.J., Udagawa, N., Elliott, J., Grail, D., Okamura, H., Kurimoto, M., Dunn, A.R., Martin, T., Gillespie, M.T., 1998. Interleukin 18 inhibits osteoclast formation via T cell production of granulocyte macrophage colony-stimulating factor. J. Clin. Invest. 101, 595e603.
Jin, C., Flavell, R.A., 2010. Molecular mechanism of NLRP3 inflammasome activation.
J. Clin. Immunol. 30, 628e631.
Kanneganti, T.D., Lamkanfi, M., 2013. K þ drops tilt the NLRP3 inflammasome.
Immunity 38, 1085e1088.
Ke, W., Xue, Z., Kai, Z., Yong, Y., Miao, Z., Tan, C., Zhou, F., Ling, Z., 2017. Puerarin inhibits amyloid b-induced NLRP3 inflammasome activation in retinal pigment epithelial cells via suppressing ROS-dependent oxidative and endoplasmic re-ticulum stresses. Exp. Cell Res. 357, 335e340.
Kiichi, N., Adam, H.J., Rathinam Vijay, A.K., Seon-Jin, L., Tamas, D., Lam, H.C., Englert, J.A., Marlene, R., Manuela, C., Pyo, K.H., 2011. Autophagy proteins regulate innate immune response by inhibiting NALP3 inflammasome-mediated mitochondrial DNA release. Nat. Immunol. 12, 222.
Kostro, K., Dudek, K., Lisiecka, U., Majerdziedzic, B., Aleksiewicz, R., Lutnicki, K., 2012. Concentrations of proinflammatory mediators of the arachidonic acid cascade in serum of sheep with natural zearalenone intoxication. Bull. Veteri-nary Inst. Pulawy 56, 75e81.
Kruidenier, L., Kuiper, I., Lamers, C.B., Verspaget, H.W., 2003. Intestinal oxidative damage in inflammatory bowel disease: semi-quantification, localization, and association with mucosal antioxidants. J. Pathol. 201, 28e36.
Kwon, K.H., Murakami, A., Ohigashi, H., 2004. Suppressive effects of natural and synthetic agents on dextran sulfate sodium-induced interleukin-1beta release from murine peritoneal macrophages. Biosci. Biotechnol. Biochem. 68, 436.
Kwon, K.H., Murakami, A., Hayashi, R., Ohigashi, H., 2005. Interleukin-1beta targets interleukin-6 in progressing dextran sulfate sodium-induced experimental
colitis. Biochem. Biophys. Res. Commun. 337, 647e654.
Lamkanfi, M., Dixit, V.M., 2012. Inflammasomes and their roles in health and dis-ease. Annu. Rev. Cell & Dev. Biol. 28, 137.
Lu, A., Magupalli, V.G., Ruan, J., Yin, Q., Atianand, M.K., Vos, M.R., Schroder,€ G.F., Fitzgerald, K.A., Wu, H., Egelman, E.H., 2014. Unified polymerization mechanism for the assembly of ASC-dependent inflammasomes. Cell 156, 1193.
Mary, V.S., Theumer, M.G., Arias, S.L., Rubinstein, H.R., 2012. Reactive oxygen species sources and biomolecular oxidative damage induced by aflatoxin B1 and fumonisin B1 in rat spleen mononuclear cells. Toxicology 302, 299.
Masters, S.L., Dunne, A., Subramanian, S.L., Hull, R.L., Tannahill, G.M., Sharp, F.A., Becker, C., Franchi, L., Yoshihara, E., Chen, Z., 2010. Activation of the Nlrp3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1b in type 2 diabetes. Nat. Immunol. 11, 897.
Okamoto, M., Liu, W., Luo, Y., Tanaka, A., Cai, X., Norris, D.A., Dinarello, C.A., Fujita, M., 2010. Constitutively active inflammasome in human melanoma cells mediating autoinflammation via caspase-1 processing and secretion of inter-leukin-1beta. J. Biol. Chem. 285, 6477.
Pinton, P., Oswald, I.P., 2014. Effect of deoxynivalenol and other Type B trichothe-cenes on the intestine: a review. Toxins 6, 1615.
Pistol, G.C., Braicu, C., Motiu, M., Gras, M.A., Marin, D.E., Stancu, M., Calin, L., Israelroming, F., Berindanneagoe, I., Taranu, I., 2015. Zearalenone mycotoxin affects immune mediators, MAPK signalling molecules, nuclear receptors and genome-wide gene expression in pig spleen. PLoS One 10, e0127503.
Prosperini, A., Juan-García, A., Font, G., Ruiz, M.J., 2013. Reactive oxygen species involvement in apoptosis and mitochondrial damage in Caco-2 cells induced by enniatins A, A 1, B and B 1. Toxicol. Lett. 222, 36.
Reddy, B.N., Raghavender, C.R., 2007. Outbreaks of aflatoxicoses in India. Afr. J. Food Agric. Nutr. Dev. 7.
Robert, H., Payros, D., Pinton, P., Theodorou, V., Mercierbonin, M., Oswald, I.P., 2017. Impact of mycotoxins on the intestine: are mucus and microbiota new targets? J. Toxicol. Environ. Health Part B Crit. Rev. 1.
Schieber, M., Chandel, N.S., 2014. ROS function in redox signaling and oxidative stress. Curr. Biol. Cb 24, R453.
Siegmund, B., Fantuzzi, G., Rieder, F., Gamboni-Robertson, F., Lehr, H.A., Hartmann, G., Dinarello, C.A., Endres, S., Eigler, A., 2001. Neutralization of interleukin-18 reduces severity in murine colitis and intestinal IFN-gamma and TNF-alpha production. Am. J. Physiol. Regul. Integr. Comp. Physiol. 281, R1264.
Stoev, S.D., 2015. Foodborne mycotoxicoses, risk assessment and underestimated hazard of masked mycotoxins and joint mycotoxin effects or interaction. En-viron. Toxicol. Pharmacol. 39, 794.
Sutterwala, F.S., Ogura, Y., Szczepanik, M., Lara-Tejero, M., Lichtenberger, G.S., Grant, E.P., Bertin, J., Coyle, A.J., Galan, J.E., Askenase, P.W., 2006. Critical role for NALP3/CIAS1/cryopyrin in innate and adaptive immunity through its regulation of Caspase-1. Immunity 24, 317.
Tschopp, J., Schroder, K., 2010. NLRP3 inflammasome activation: the convergence of multiple signalling pathways on ROS production? Nat. Rev. Immunol. 10, 210e215.
Vergauwen, H., 2015. The IPEC-J2 Cell Line. Springer International Publishing. Walsh, A.J., Ghosh, A., Brain, A.O., Buchel, O., Burger, D., Thomas, S., White, L.,
Collins, G.S., Keshav, S., Travis, S.P., 2014. Comparing disease activity indices in ulcerative colitis. J. Crohns Colitis 8, 318.
Wan, L.Y., Woo, C.S., Turner, P.C., Wan, J.M., El-Nezami, H., 2013. Individual and combined effects of Fusarium toxins on the mRNA expression of pro-inflammatory cytokines in swine jejunal epithelial cells. Toxicol. Lett. 220, 238e246.
Wan, L.-Y.M., Allen, K.J., Turner, P.C., El-Nezami, H., 2014. Modulation of mucin mRNA (MUC5AC and MUC5B) expression and protein production and secretion in caco-2/HT29-MTX Co-cultures following exposure to individual and com-bined Fusarium mycotoxins. Toxicol. Sci. 139, 83e98.
Yao, J., Wang, J.Y., Liu, L., Li, Y.X., Xun, A.Y., Zeng, W.S., Jia, C.H., Wei, X.X., Feng, J.L., Zhao, L., 2010. Anti-oxidant effects of resveratrol on mice with DSS-induced ulcerative colitis. Arch. Med. Res. 41, 288e294.
Zaki, M.H., Lamkanfi, M., Kanneganti, T.D., 2011. The Nlrp3 inflammasome: contri-butions to intestinal homeostasis. Trends Immunol. 32, 171e179.
Zhou, R., Tardivel, A., Thorens, B., Choi, I., Tschopp, J., 2010. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat. Immunol. 11, 136.
Zmora, N., Levy, M., Pevsnerfishcer, M., Elinav, E., 2017. Inflammasomes and intes-tinal inflammation. Mucosal Immunol. 10, 865e883.