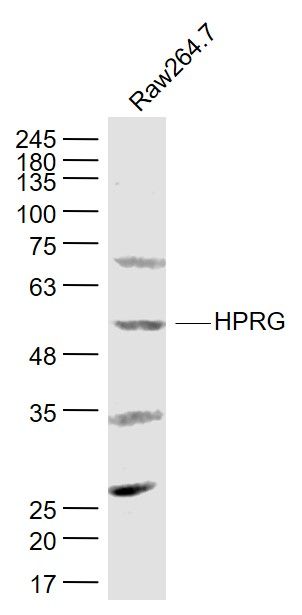
产品货号 : mlR6052
英文名称 : HPRG
中文名称 : 组氨酸富含脯氨酸糖蛋白抗体
别 名 : Histidine proline rich glycoprotein; Histidine rich glycoprotein; HPRG; HRGP; Thrombophilia due to elevated HRG.
研究领域 : 心血管 生长因子和激素
抗体来源 : Rabbit
克隆类型 : Polyclonal
交叉反应 : Human, Mouse, Rat,
产品应用 : WB=1:500-2000 ELISA=1:500-1000 IHC-P=1:400-800 IHC-F=1:400-800 IF=1:100-500 (石蜡切片需做抗原修复)
not yet tested in other applications.
optimal dilutions/concentrations should be determined by the end user.
分 子 量 : 58kDa
细胞定位 : 分泌型蛋白
性 状 : Lyophilized or Liquid
浓 度 : 1mg/ml
免 疫 原 : KLH conjugated synthetic peptide derived from human HPRG/HRG:75-180/525
亚 型 : IgG
纯化方法 : affinity purified by Protein A
储 存 液 : 0.01M TBS(pH7.4) with 1% BSA, 0.03% Proclin300 and 50% Glycerol.
保存条件 : Store at -20 °C for one year. Avoid repeated freeze/thaw cycles. The lyophilized antibody is stable at room temperature for at least one month and for greater than a year when kept at -20°C. When reconstituted in sterile pH 7.4 0.01M PBS or diluent of antibody the antibody is stable for at least two weeks at 2-4 °C.
PubMed : PubMed
产品介绍background:
HRG contains two cystatin-like domains and is located in plasma and platelets. The physiological function is not yet known. It binds heme, dyes and divalent metal ions. It can inhibit rosette formation and interacts with heparin, thrombospondin, and the lysine-binding site of plasminogen. A potential prothrombotic effect of HRG is indicated by the inhibition of fibrinolysis and the reduction of inhibition of coagulation. Mutations in HRG lead to thrombophilia due to abnormal histidine-rich glycoprotein levels.
Function:
Plasma glycoprotein that binds a number of ligands such as heme, heparin, heparan sulfate, thrombospondin, plasminogen, and divalent metal ions. Binds heparin and heparin/glycosaminoglycans in a zinc-dependent manner. Binds heparan sulfate on the surface of liver, lung, kidney and heart endothelial cells. Binds to N-sulfated polysaccharide chains on the surface of liver endothelial cells. Inhibits rosette formation. Acts as an adapter protein and is implicated in regulating many processes such as immune complex and pathogen clearance, cell chemotaxis, cell adhesion, angiogenesis, coagulation and fibrinolysis. Mediates clearance of necrotic cells through enhancing the phagocytosis of necrotic cells in an heparan sulfate-dependent pathway. This process can be regulated by the presence of certain HRG ligands such as heparin and zinc ions. Binds to IgG subclasses of immunoglobins containing kappa and lambda light chains with different affinities regulating their clearance and inhibiting the formation of insoluble immune complexes. Tethers plasminogen to the cell surface. Binds T-cells and alters the cell morphology. Modulates angiogenesis by blocking the CD6-mediated antiangiongenic effect of thrombospondins, THBS1 and THBS2. Acts as a regulator of the vascular endothelial growth factor (VEGF) signaling pathway; inhibits endothelial cell motility by reducing VEGF-induced complex formation between PXN/paxillin and ILK/integrin-linked protein kinase and by promoting inhibition of VEGF-induced tyrosine phosphorylation of focal adhesion kinases and alpha-actinins in endothelial cells. Also plays a role in the regulation of tumor angiogenesis and tumor immune surveillance. Normalizes tumor vessels and promotes antitumor immunity by polarizing tumor-associated macrophages, leading to decreased tumor growth and metastasis.
Subunit:
Interacts (via the HRR domain) with TPM1; the interaction appears to contribute to the antiangiogenic properties of the HRR domain. Interacts with THBS2; the interaction blocks the antiangiogenic effect of THBS2 with CD36 (By similarity). Interacts with THBS1 (via the TSP type I repeats); the interaction blocks the antiangiogenic effect of THBS1 with CD3. Interacts with PLG (via its Kringle domains); the interaction tethers PLG to the cell surface and enhances its activation. Interacts with HPSE; the interaction is enhanced at acidic pH, partially inhibits binding of HPSE to cell surface receptors and modulates its enzymatic activity. Interacts (via the HRR domain) with TMP1; the interaction partially mediates the antiangiogenic properties of HRG. Interacts with kappa and lambda light chains of IgG molecules. Interacts with ATP5A1; the interaction occurs on the surface of T-cells and alters their cell morphology in concert with CONA. Binds IgG molecules containing kappa and lambda light chains and inhibits the formation of insoluble immunoglobulin complexes. Interacts with F12; the interaction, which is enhanced in the presence of zinc ions and inhibited by heparin-binding to HRG, inhibits factor XII autoactivation and contact-initiated coagulation.
Subcellular Location:
Secreted.
Tissue Specificity:
Expressed in macrophages and in malignant cells. Expressed by the liver and secreted in plasma (at protein level)
Post-translational modifications:
Proteolytic cleavage produces several HRG fragments which are mostly disulfide-linked and, therefore, not released. Cleavage by plasmin is inhibited in the presence of heparin, zinc ions or in an acidic environment. Cleavage reduces binding of HRG to heparan sulfate, but enhances the ability of HRG to bind and tether plasminogen to the cell surface. On platelet activation, releases a 33 kDa antiangiogenic peptide which encompasses the HRR. Also cleaved in the C-terminal by plasmin.
N-glycosylated.
DISEASE:
Defects in HRG are the cause of thrombophilia due to histidine-rich glycoprotein deficiency (THPH11) [MIM:613116]. A hemostatic disorder characterized by a tendency to thrombosis.
Similarity:
Contains 2 cystatin domains.
SWISS:
P04196
Gene ID:
3273
Important Note:
This product as supplied is intended for research use only, not for use in human, therapeutic or diagnostic applications.
产品图片 :